Atomic Number 56 Belongs To Which Block
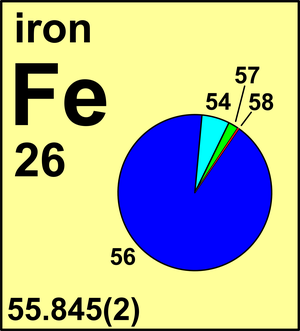
Barium has an atomic number of 56, which means that it has 56 protons. Thus, to have a neutral charge, it must carry 56 electrons as well. The atomic mass of Barium is 137.33, which means that the. Barium (Ba) is a soft silvery white coloured metal that has the atomic number 56 in the periodic table. It is an Alkaline earth metal and is located in Group 2 of the periodic table. It has the symbol Ba. Canon mf5940dn driver for mac. Isotope: Mass: Abundance: Spin: Mag Moment: 130 Ba: 129.906282: 0.11%: 0: 132 Ba: 131.905042: 0.10%: 0: 134 Ba: 133.904486: 2.42%: 0: 135 Ba: 134.905665: 6.59. Rank the following elements from smallest to largest electronegativity based on the trends you have discovered thus far in the periodic table: barium (atomic number 56), bromine (atomic number 35), and iron (atomic number 26). Iron-56 (56 Fe) is the most common isotope of iron. About 91.754% of all iron is iron-56. Of all nuclides, iron-56 has the lowest mass per nucleon. With 8.8 MeV binding energy per nucleon, iron-56 is one of the most tightly bound nuclei.
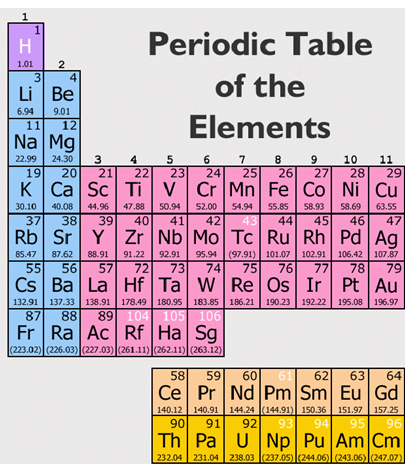
In the modern periodic table, the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that determines most of the chemical behavior of an element.
In a periodic table arranged in order of increasing atomic number, elements having similar chemical properties naturally line up in the same column (group). For instance, all of the elements in Group 1A are relatively soft metals, react violently with water, and form 1+ charges; all of the elements in Group 8A are unreactive, monatomic gases at room temperature, etc. In other words, there is a periodic repetition of the properties of the chemical elements with increasing mass.

Atomic Number 56 Mass Number 137
In the original periodic table published by Dimitri Mendeleev in 1869, the elements were arranged according to increasing atomic mass— at that time, the nucleus had not yet been discovered, and there was no understanding at all of the interior structure of the atom, so atomic mass was the only guide to use. Once the structure of the nucleus was understood, it became clear that it was the atomic number that governed the properties of the elements.
