How to calculate the number of electrons in an ion
- Hydrogen has 1 electron each. So that adds up to 2 electrons from Hydrogen. Next Oxygen has a total of 8 electrons. This adds up to a total of 10 electrons!
- Valence electrons are the electrons contained in the outermost shell. If you look at the periodic table and at the period numbers, that is the number of valence electrons. If the number is larger than 10, subtract 10 so you get two valence electrons. Example: Oxygen is in the 16th period.
- Generally, the number of electrons—alongside with protons and neutrons—in an atom can be determined from a set of simple rules. The number of protons in the nucleus of the atom is equal to the atomic number (Z).
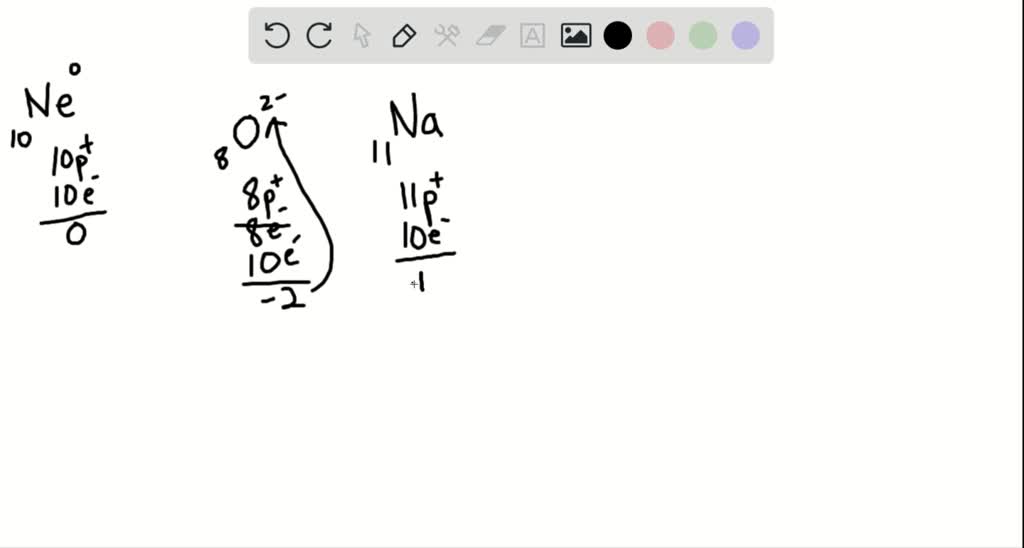
To calculate the number of electrons in an ion that carries a positive charge, you must recall that when an ion carries a positive charge, the number of electrons are fewer than the number of protons. So to get the number of electrons, you must subtract the size of charge (which is often written as a superscript on the right side of the symbol) from the atomic number or proton number. Let’s use figure 2 as an example. As you can see, Mg has an atomic number of 12 and a charge of +2, so to get the number of electrons for Mg ion, we must subtract 2 from the atomic number. Thus, 12-2= 10 electrons.
In this video we’ll use the Periodic table and a few simple rules to find the number of protons and electrons for the Beryllium ion (Be2+). From the Periodic. Number of Electrons - How to find the Number of Electrons - Examples The atomic number is based on the number of Protons in the atom of an element. (Note: Atoms must also have equal numbers of Electrons & Protons.) So, if we know the atomic number of an element then we also know how many Protons are in an element and therefore the number of electrons in an element.
Likewise, to calculate the number of electrons for an ion that carries a negative charge, you must recall that when an ion carries a negative charge, the number of electrons are more than the number of protons. So to get the number of electrons, you must add the size of charge to the atomic or proton number. So from figure 3, the number of electrons for chloride ion is 17 + 1 = 18 electrons.
To learn how atoms form ions, click here.
To learn more about subatomic particles, click here
To learn why a cation is smaller than its neutral atom, click here
How many electrons are in #n=3#, #l= 2#?
1 Answer
Explanation:
The idea here is that you must use the value of the angular momentum quantum number,
The number of values that the magnetic quantum number can take tells you the number of orbitals that are present in a given subshell.
So, you know that the magnetic quantum number depends on the value of the angular momentum quantum number
In your case, you have
Number Of Electrons



This tells you that the
Since each orbital can hold a maximum of
Therefore, a maximum number of
Number Of Electrons In Sodium
How Do You Find Number Of Electrons
These electrons are located on the third energy level, in the
Related questions
